
HartRAO Home > Spectra Contents > Spectra Introduction > Recombination Line Spectra
A cloud of gas in which the hydrogen and carbon atoms have lost single electrons after being hit by ultraviolet light from an O- or B-type star is called ionized gas. The free electrons in the ionized gas then recombine with the atoms. After recombining the atoms are initially in highly excited states. They then jump down the energy level "ladder" to less excited states. As each jump occurs energy is emitted. This energy appears as emission lines at specific wavelengths. For energy levels around 100, these lines occur in the radio part of the electromagnetic spectrum. Lines at optical wavelengths are emitted when the electron is almost down to the ground state, at the bottom of the ladder. These lines gives rise to the pink glow seen in photographs of emission nebulae.
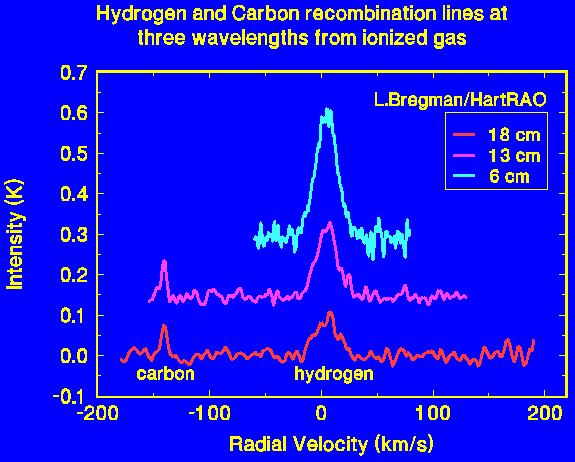
The lines shown here were all observed from the same HII region, but at three different wavelengths. The hydrogen lines increase in strength as the wavelength gets shorter. The temperature of the gas can be deduced from the intensity of the line, its width, and the intensity of the continuum emission from the gas. In this case the temperature is about 7000 K, which is typical of ionized hydrogen regions.
The carbon lines seen here are unusually strong. They arise in a warm (1000 K) interface between the main ionized hydrogen cloud and a molecular cloud on the side facing us. The carbon lines are shifted in frequency relative to the hydrogen owing to the greater mass of the carbon atom, twelve times that of hydrogen. The velocity scale on the image is relative to the hydrogen; the carbon gas in fact has the same velocity as the hydrogen.
The carbon lines are much narrower than the hydrogen lines. This is a consequence of the lower temperature of the gas containing the carbon and of the greater mass of the carbon atoms, which is twelve times that of the hydrogen atoms.
These spectra were obtained by Leigh Bregmann, a student at the University of the Witwatersrand, as part of a third year Physics project.